Meanwhile at the CDC…
We don’t have enough vaccine yet for all health care workers. We will eventually, but we don’t yet.
Helen Keipp Talbot — who is known by her middle name — raised serious concerns during the meeting of the Advisory Committee on Immunization Practices about using the vaccines in the frail elderly, noting there are no data yet to suggest the vaccines work in this population.
CDC advisory panel’s lone dissenter on why long-term care residents shouldn’t receive Covid-19 vaccine first — https://www.statnews.com/2020/12/03/cdc-advisory-panels-lone-dissenter-on-why-long-term-care-residents-shouldnt-receive-covid-19-vaccine-first/
Talbot is an associate professor of infectious diseases at Vanderbilt University. Despite the fact no one seems to be listening to her opinions, she makes some excellent observations.
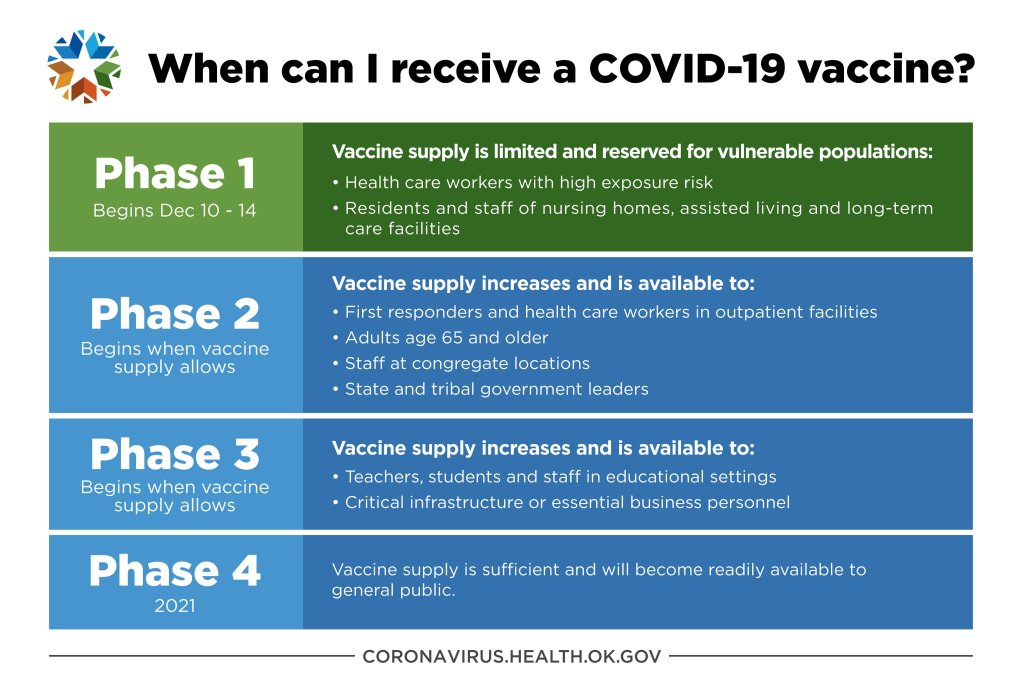
Meanwhile back in Oklahoma




You must be logged in to post a comment.