The Centers for Disease Control and Prevention (CDC) recommends that the second dose of the COVID-19 vaccine be given within 3 weeks of the first dose for the Pfizer vaccine and within 4 weeks for the Moderna vaccine. No more than 6 weeks should lapse between doses, although if the second dose is not given during these time frames, it can be given without the need to repeat the first dose. It is not recommended to give the second dose any earlier than stated above, but if a person needs to get the second dose earlier, giving the second dose up to 4 days ahead of schedule is allowed.
Necessity of 2 Doses of the Pfizer and Moderna COVID-19 Vaccines — https://jamanetwork.com/journals/jama/fullarticle/2776229
Pharma
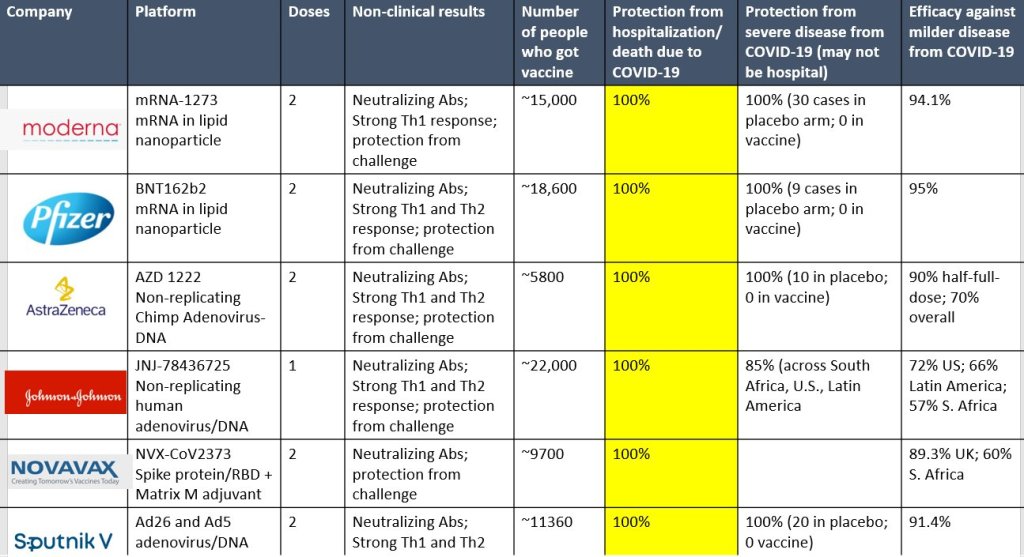
Just Another SARS-CoV-2 Vaccine Chart
Doctors keep making cool graphics. I keep finding them. Note Column six from the left in yellow.
Monica Gandhi MD, MPH is Professor of Medicine and Associate Division Chief (Clinical Operations/ Education) of the Division of HIV, Infectious Diseases, and Global Medicine at UCSF/ San Francisco General Hospital. She also serves as the Director of the UCSF Center for AIDS Research (CFAR) and the Medical director of the HIV Clinic at SFGH (“Ward 86”). Dr. Gandhi completed her M.D. at Harvard Medical School and then came to UCSF in 1996 for residency training in Internal Medicine. After her residency, Dr. Gandhi completed a fellowship in Infectious Diseases and a postdoctoral fellowship at the Center for AIDS Prevention Studies, both at UCSF. She also obtained a Masters in Public Health from Berkeley in 2001 with a focus on Epidemiology and Biostatistics.
https://profiles.ucsf.edu/monica.gandhi
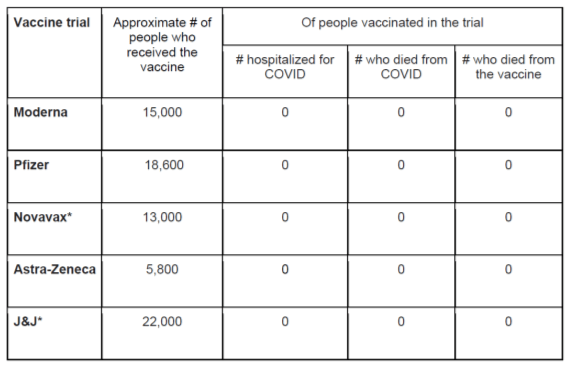
Love the Zeroes
Ashish K. Jha, MD, MPH, is a physician, health policy researcher, and the third Dean of the Brown University School of Public Health. Before joining Brown, he was the K.T. Li Professor of Global Health at the Harvard T.H. Chan School of Public Health and Director of the Harvard Global Health Institute (HGHI).
https://www.brown.edu/academics/public-health/about/people/dean/ashish-jha
Quote for Today – 01.30.21
“In the event that you have the choice to get vaccinated, I’d encourage you to take the vaccine that you’re given,” John Brooks, the chief medical officer of the Centers for Disease Control and Prevention’s Covid-19 response, said at a briefing Friday.
Additional Covid-19 vaccines bring choices — and complications — to the rollout — https://www.statnews.com/2021/01/29/additional-covid19-vaccines-bring-choices-and-complications-to-rollout/
Let’s get one thing clear: I am not a medical doctor nor is anything you read on this blog to be considered medical advice. Now that we have mutual understanding…
This is NO time to be picky. You can be picky about the brand of coffee you drink (or how it should be brewed). Or you can be picky about which vegan eatery serves up the best tofu in your town. Go ahead. Be picky on pretty much anything in your life.
But when it comes down to a vaccine for a virus that to the best of our knowledge we have no known natural immunity I suggest you take whatever vaccine is available.
Leave the debates about efficacy to experts who know what they’re talking about.
Rant over.
Indian scientists divided over restricted use approval for Covaxin — Science Chronicle

While some scientists have raised concerns about granting restricted use approval to Covaxin even in the absence of efficacy data, four-dozen scientists have in a statement slammed them saying “reprehensible utterances are causing huge credibility crisis for the Indian scientific community”. Apparently, questioning the approval process by the Indian regulator is seen as being anti-Indian […]
Indian scientists divided over restricted use approval for Covaxin — Science Chronicle
India’s drug regulator approved two COVID-19 vaccines on 3 January, a decision Prime Minister Narendra Modi hailed on Twitter as “a decisive turning point to strengthen a spirited fight!” against the pandemic and a testament to the Indian scientific community’s self reliance. But some scientists and patient advocates are sharply critical of the move—in particular, the decision to greenlight Covaxin, a vaccine developed in India by Bharat Biotech, without awaiting the results of a phase III trial to determine efficacy and safety…
Efficacy data from a challenge study in rhesus macaques and immune responses in a human phase II trial suggested the vaccine was likely to be very effective.
The approval of a vaccine without phase III data is “unconscionable,” says Vineeta Bal, an immunologist at India’s National Institute of Immunology.
Scientists criticize ‘rushed’ approval of Indian COVID-19 vaccine without efficacy data — https://www.sciencemag.org/news/2021/01/scientists-criticize-rushed-approval-indian-covid-19-vaccine-without-efficacy-data
It worked in monkeys so let’s just skip the Phase III trial.
See my earlier post I would not take Covaxin without efficacy data: Gagandeep Kang — Science Chronicle

Utter Chaos? No Just Your Normal Vaccine Rollout in a Pandemic
With the vaccine rollout left mostly up to states and counties, they have had to rapidly devise their own methods for distributing shots to their residents. Every state has its own priority system and way of scheduling appointments, which sometimes change week to week. The complicated logistics paired with inconsistent communication to the public has resulted in mass confusion. The result: People are spending hours seeking information and searching for coveted appointment slots.
‘Just utter chaos’: A Twitter thread offers a window into the frustrating search for Covid-19 shots — https://www.statnews.com/2021/01/28/just-utter-chaos-twitter-thread-offers-window-into-frustrating-search-for-covid19-shots/?utm_campaign=rss
Here in Oklahoma we’re in Phase 2 of the rollout and the process to get a Covid vaccination appointment in this state can best be described as incredibly difficult. We don’t have a huge number of residents here in flyover country and to be honest, that’s a good thing. I can’t imagine how difficult this process is in the more heavily populated areas of the country.
BTW I’m getting jabbed tomorrow.

Vinay Prasad MD MPH is a Very Smart Person
For most people, once you get 14 days out of your second dose of vaccine, I believe you can ease up on masking or another restriction, such as visiting a loved one for lunch or having more than one person visit a nursing home at the same time, or a small gathering of vaccinated people for dinner without masks.
Op-Ed: Throw Away Your Mask After COVID Vaccination? — Op-Ed: Throw Away Your Mask After COVID Vaccination?
Dr. Prasad’s Op-Ed article is worth reading. Or if you’re a watch, listen and learn type check out the video.
BUT if you have an hour to spare the following podcast is downright entertaining.
Update on Severe Allergic or Anaphylactic Reactions to Initial Dose of Pfizer COVID-19 Vaccine — The Skeptical Cardiologist
A report from two CDC scientists was published online today in JAMA Insights which describes in detail 21 cases of anaphylaxis that were reported to the US Vaccine Adverse Events Reporting System between December 14-23, 2020. This corresponds to a very low rate of 11.1 cases of anaphylaxis per million doses administered. 17 of these…
I continue to urge all my patients to get vaccinated as soon as possible. Benefits far outweigh the risk.
Update on Severe Allergic or Anaphylactic Reactions to Initial Dose of Pfizer COVID-19 Vaccine — The Skeptical Cardiologist
ACE2-interacting domain of SARS-CoV-2
In a study published in the Journal of Neuroimmune Pharmacology, mouse models with COVID-19 showed positive results when a small peptide was introduced nasally. The peptide proved effective in reducing fever, protecting the lungs, improving heart function and reversing cytokine storm — a condition in which an infection triggers the immune system to flood the bloodstream with inflammatory proteins. The researchers also report success in preventing the disease from progression.
Rush University Medical Center. “Potential COVID-19 drug is successful in lab study: Peptide reduced COVID-19 symptoms in mice.” ScienceDaily. http://www.sciencedaily.com/releases/2021/01/210119194322.htm (accessed January 20, 2021).
Journal Reference – Ramesh K. Paidi, Malabendu Jana, Rama K. Mishra, Debashis Dutta, Sumita Raha, Kalipada Pahan. ACE-2-interacting Domain of SARS-CoV-2 (AIDS) Peptide Suppresses Inflammation to Reduce Fever and Protect Lungs and Heart in Mice: Implications for COVID-19 Therapy. Journal of Neuroimmune Pharmacology, 2021; DOI: 10.1007/s11481-020-09979-8
Our neighbor Dr. Arlan Richardson at https://nathanshockcenters.org/oklahoma part of the University of Oklahoma Health Sciences Center knows a lot about mice. I’ll have to ask him what he thinks of the potential of this peptide for human use.
J&J Covid-19 Vaccine Appears to be Safe
A total of 800 adults aged 18 to 55 or aged 65 and up were randomized to various combinations of low-dose or high-dose vaccines or placebo, given 56 days apart.
Adverse events were common, with fatigue, headache, myalgia, and injection-site pain reported most often. At day 29 after the first dose, the seroconversion rate was 99% or more in the younger cohort across dosing groups. Older vaccine recipients had a 96% seroconversion rate. At 57 days after the first dose, antibody titers had increased further.
COVID-19: Single Dose of J&J Vaccine / Plasma / New Testing Requirement — https://www.jwatch.org/fw117413/2021/01/13/covid-19-single-dose-j-j-vaccine-plasma-new-testing
The J&J vaccine is in a multi-center, placebo-controlled, phase 1–2a trial. Here’s the link to the NEJM article https://www.nejm.org/doi/full/10.1056/NEJMoa2034201
From Stat Phase 3 results are coming soon.
There are two Phase 3 studies running. A 40,000-volunteer study of the one-dose vaccine, conducted in the U.S., is set to read out in the next two weeks. A second, equally big study is being conducted using the same vaccine given as two doses, each administered 57 days apart, in case the vaccine does not prove effective in a one-dose regimen or there are other advantages, such as the durability of the vaccine, to giving a second dose.
https://www.statnews.com/2021/01/13/data-fuel-debate-over-whether-jjs-one-dose-covid-vaccine-will-measure-up/ — Data fuel debate over whether J&J’s one-dose Covid vaccine will measure up
And from the Medscape article.
Unlike the Pfizer/BioNTech and Moderna messenger RNA vaccines, the Johnson & Johnson product is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein.
The Next Likely COVID-19 Vaccine Has Its Advantages — https://www.medscape.com/viewarticle/944151?src=rss
You must be logged in to post a comment.