FDA’s Vaccine & Related Biological Products Advisory Committee has voted unanimously (22-0) that benefits of Janssen’s single-dose #COVID19 #vaccine outweigh risks for ages 18y & up.
— Tatiana Prowell, MD (@tmprowell) February 26, 2021
Per company, study in adolescents to open soon w/ younger children to follow.#regulatory pic.twitter.com/Uk78rQuQcN
vaccine
Meanwhile in the UK…
I’ve been having a lot of fun since learning how to embed a Bird App Tweet into my posts.
Vaccination Lessons from Chick-fil-A
What if we partnered with this restaurant chain and opened drive-thru vaccination sites on Sundays leasing locations and staff?
A Ph.D. microbiologist and immunologist, Bauman understands the best experiments don’t start from scratch. So, he decided to emulate the most efficient, customer-focused operation he knew. He went to the Chick-fil-A drive-through in Norman and recorded the entire process. Then, he did his best to duplicate it (minus the waffle fries and dipping sauce).
“Once you come in, you should never stop in line — just like Chick-fil-A,” Bauman said. IMMY staff walked alongside each person who entered, checking them in with iPads as they guided them toward the ballroom where nurses gave shots. “The only time you should stop is when you put your butt in the seat to get the vaccine.”
Vaccination lessons from Chick-fil-A (hold the fries) – https://oklahoman.com/article/5682352/vaccination-lessons-from-chick-fil-a-hold-the-fries
That Second Vaccine Shot – ZDoggMD
Just Another SARS-CoV-2 Vaccine Chart – Updated 02.11.21
Doctors keep making cool graphics. I keep finding them. Note Column six from the left in yellow has been updated.

Monica Gandhi MD, MPH is Professor of Medicine and Associate Division Chief (Clinical Operations/ Education) of the Division of HIV, Infectious Diseases, and Global Medicine at UCSF/ San Francisco General Hospital. She also serves as the Director of the UCSF Center for AIDS Research (CFAR) and the Medical director of the HIV Clinic at SFGH (“Ward 86”). Dr. Gandhi completed her M.D. at Harvard Medical School and then came to UCSF in 1996 for residency training in Internal Medicine. After her residency, Dr. Gandhi completed a fellowship in Infectious Diseases and a postdoctoral fellowship at the Center for AIDS Prevention Studies, both at UCSF. She also obtained a Masters in Public Health from Berkeley in 2001 with a focus on Epidemiology and Biostatistics.
https://profiles.ucsf.edu/monica.gandhi
Necessity of 2 Doses of the Pfizer and Moderna COVID-19 Vaccines – JAMA
The Centers for Disease Control and Prevention (CDC) recommends that the second dose of the COVID-19 vaccine be given within 3 weeks of the first dose for the Pfizer vaccine and within 4 weeks for the Moderna vaccine. No more than 6 weeks should lapse between doses, although if the second dose is not given during these time frames, it can be given without the need to repeat the first dose. It is not recommended to give the second dose any earlier than stated above, but if a person needs to get the second dose earlier, giving the second dose up to 4 days ahead of schedule is allowed.
Necessity of 2 Doses of the Pfizer and Moderna COVID-19 Vaccines — https://jamanetwork.com/journals/jama/fullarticle/2776229
Just Another SARS-CoV-2 Vaccine Chart
Doctors keep making cool graphics. I keep finding them. Note Column six from the left in yellow.
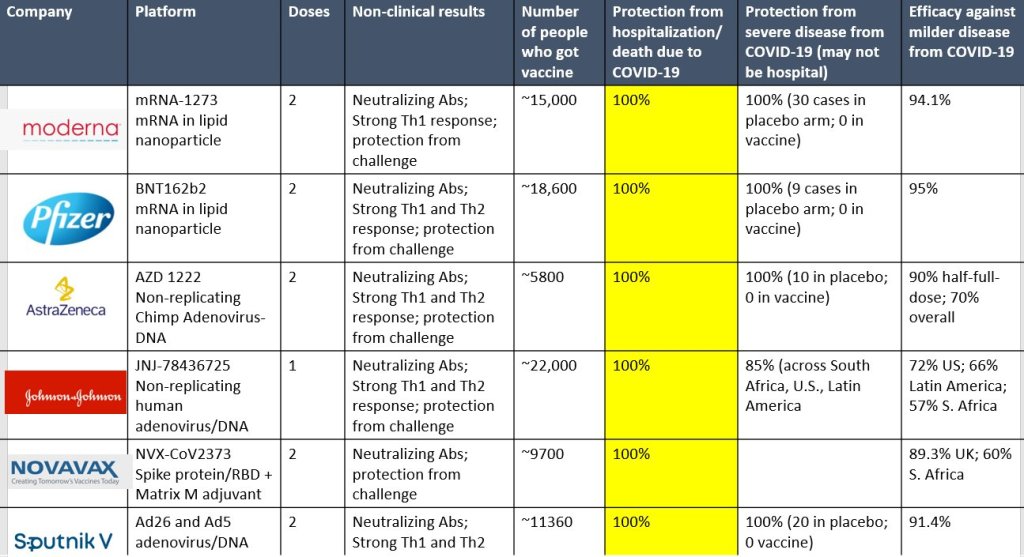
Monica Gandhi MD, MPH is Professor of Medicine and Associate Division Chief (Clinical Operations/ Education) of the Division of HIV, Infectious Diseases, and Global Medicine at UCSF/ San Francisco General Hospital. She also serves as the Director of the UCSF Center for AIDS Research (CFAR) and the Medical director of the HIV Clinic at SFGH (“Ward 86”). Dr. Gandhi completed her M.D. at Harvard Medical School and then came to UCSF in 1996 for residency training in Internal Medicine. After her residency, Dr. Gandhi completed a fellowship in Infectious Diseases and a postdoctoral fellowship at the Center for AIDS Prevention Studies, both at UCSF. She also obtained a Masters in Public Health from Berkeley in 2001 with a focus on Epidemiology and Biostatistics.
https://profiles.ucsf.edu/monica.gandhi
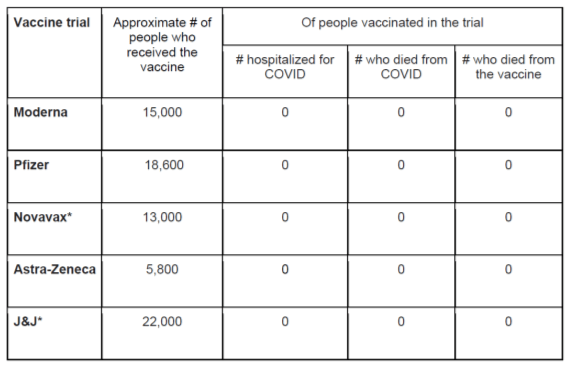
Love the Zeroes
Ashish K. Jha, MD, MPH, is a physician, health policy researcher, and the third Dean of the Brown University School of Public Health. Before joining Brown, he was the K.T. Li Professor of Global Health at the Harvard T.H. Chan School of Public Health and Director of the Harvard Global Health Institute (HGHI).
https://www.brown.edu/academics/public-health/about/people/dean/ashish-jha
Quote for Today – 01.30.21
“In the event that you have the choice to get vaccinated, I’d encourage you to take the vaccine that you’re given,” John Brooks, the chief medical officer of the Centers for Disease Control and Prevention’s Covid-19 response, said at a briefing Friday.
Additional Covid-19 vaccines bring choices — and complications — to the rollout — https://www.statnews.com/2021/01/29/additional-covid19-vaccines-bring-choices-and-complications-to-rollout/
Let’s get one thing clear: I am not a medical doctor nor is anything you read on this blog to be considered medical advice. Now that we have mutual understanding…
This is NO time to be picky. You can be picky about the brand of coffee you drink (or how it should be brewed). Or you can be picky about which vegan eatery serves up the best tofu in your town. Go ahead. Be picky on pretty much anything in your life.
But when it comes down to a vaccine for a virus that to the best of our knowledge we have no known natural immunity I suggest you take whatever vaccine is available.
Leave the debates about efficacy to experts who know what they’re talking about.
Rant over.
Indian scientists divided over restricted use approval for Covaxin — Science Chronicle

While some scientists have raised concerns about granting restricted use approval to Covaxin even in the absence of efficacy data, four-dozen scientists have in a statement slammed them saying “reprehensible utterances are causing huge credibility crisis for the Indian scientific community”. Apparently, questioning the approval process by the Indian regulator is seen as being anti-Indian […]
Indian scientists divided over restricted use approval for Covaxin — Science Chronicle
India’s drug regulator approved two COVID-19 vaccines on 3 January, a decision Prime Minister Narendra Modi hailed on Twitter as “a decisive turning point to strengthen a spirited fight!” against the pandemic and a testament to the Indian scientific community’s self reliance. But some scientists and patient advocates are sharply critical of the move—in particular, the decision to greenlight Covaxin, a vaccine developed in India by Bharat Biotech, without awaiting the results of a phase III trial to determine efficacy and safety…
Efficacy data from a challenge study in rhesus macaques and immune responses in a human phase II trial suggested the vaccine was likely to be very effective.
The approval of a vaccine without phase III data is “unconscionable,” says Vineeta Bal, an immunologist at India’s National Institute of Immunology.
Scientists criticize ‘rushed’ approval of Indian COVID-19 vaccine without efficacy data — https://www.sciencemag.org/news/2021/01/scientists-criticize-rushed-approval-indian-covid-19-vaccine-without-efficacy-data
It worked in monkeys so let’s just skip the Phase III trial.
See my earlier post I would not take Covaxin without efficacy data: Gagandeep Kang — Science Chronicle

You must be logged in to post a comment.