According to the USP, the bulk of the APIs come from India. That country is responsible for 50% of the active pharmaceutic ingredients. China is not far behind at 32%. The European Union supplies 10%. That’s a big change since 2000. Back then, European countries like France, Germany, Switzerland and Denmark supplied 42% of the APIs. Drug Recalls From India – Can You Trust Foreign-Made Generics? – https://www.peoplespharmacy.com/articles/more-drug-recalls-from-india-do-you-trust-foreign-made-generics
Dozens of companies received approval from the FDA over the years to sell metoprolol and bupropion in the U.S. Yet from 2018 to 2024, the agency reported running only 2 tests on metoprolol and 7 on bupropion through its quality surveillance program — in each case, by pulling a sample from a single drug maker. In many of those years, the drugs weren’t tested at all, FDA records show. Those that were assessed received passing results. The FDA Often Doesn’t Test Generic Drugs for Quality Concerns, So ProPublica Did – https://www.propublica.org/article/fda-generic-drug-testing
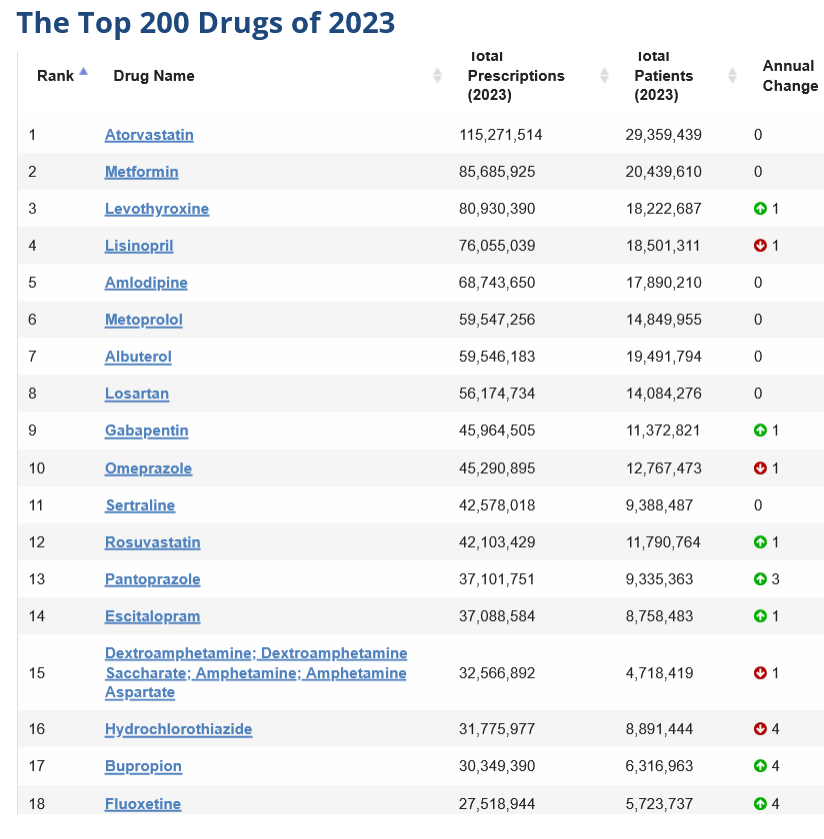
ClinCalc DrugStats Database – https://clincalc.com/DrugStats/
Both articles are long reads but worth your time.
Yikes.
So what can we do?
Great question. I think the answer/first step is awareness. Awareness by patients and physicians that some symptoms may be the result of generic formulations not working as they should due to shoddy manufacturing and lack of oversight/testing. When the problems become to big to ignore, the pressure on the FDA to act will hopefully bring about change.
In the meantime, we just have to put up with this? It is so hard to know if a drug is working let alone consider whether you have a generic that is contaminated or lacks potency. It’s unconscionable that people should have to suffer until the consequences are severe enough for the FDA to do something. Nearly all of my medications are generic.